Guest Blog Post Over at Scientific American
I’ve written a guest blog post for Scientific American, the subject of which is computational biology and using mathematical models to perform in silico experiments in the heart. Some of the content is similar to what I’ve written about before – why computational biology can be useful, etc. But I’ve also included some cool stuff that one of my labmates is doing with 3D computer models of the atria. If you’re so inclined, check it out!
There’s a lot of great stuff being continuously posted to that blog; it’s a good one to keep you eye on!
Spaceflight and the Immune System: Reduced Diversity?
I ran across an interesting post over at Astrobiology Magazine the other day (which is really just a verbatim repost of the original press release posted by the Federation of the American Societies for Experimental Biology [FASEB]). The subject of this post was a research article that was recently published online at The FASEB Journal, which can be found here (abstract can be accessed for free, but article access requires a subscription).
The focus of the article is how spaceflight affects a process called somatic hypermutation, which is involved in the generation of the antibodies that are necessary for our immune systems to recognize pathogens and eliminate them. Antibodies function as receptors that can bind to a specific pathogen, and upon doing so initiate a series of events that culminate in the pathogen’s destruction and/or eradication. However, we are not born with the complete array of antibodies necessary to cover all pathogens that we may encounter during our lifetimes, so it’s important for our immune system to possess the ability to generate new antibodies de novo. I’ve included a cartoon of an antibody below:

Image Credit: Wikimedia Commons
For the most part, all antibodies have the same structure, save for a small region near the tips where antigen (e.g. pathogen) binding takes place. A wide variety of antibodies can be generated if mutations occur in the DNA that codes for these variable regions of antibodies. Normally we don’t want mutations to occur in our DNA, as that can lead to various diseases including cancer (and our cells contain mechanisms to repair mutated/damaged DNA or self-destruct if the damage is too great), but this is a case where mutations are beneficial.
At any rate, when a B cell (one type of cell under the employ of our immune system) encounters an antigen, it is programmed to proliferate. During these cell divisions, the DNA coding for the antibody variable regions undergoes a high rate of mutation. This program of increased mutation in B cells, known as somatic hypermutation (SHM), produces the necessary antibody diversity.
In the aforementioned study, researchers immunized newts that were aboard the Mir space station, then later analyzed DNA sequences to quantify the rate at which DNA mutation in the variable antibody regions had occurred. The group of newts that were immunized in space were compared to two control groups: one in which the newts had been immunized (and maintained) on Earth, and another in which the newts were not immunized at all (and also maintained here on Earth). One of the principal findings of the study was that the space-immunized newts only exhibited about half of the somatic hypermutation that their Earth-immunized counterparts did. The implication of this finding is that prolonged spaceflight resulted in reduced antibody gene mutation, presumably resulting in lower antibody diversity, which could possibly diminish the ability of the immune system to combat infections and cancers.
The obvious next question is what caused the decreased SHM rate? Is it related to prolonged exposure to microgravity? The authors seem confident in ruling out lack of food intake, as the newts were periodically force fed and were not underweight upon their return to earth. Unfortunately, the study was not sufficiently powered to make any determinations about what the proximal cause might be, as only two of the original eight newts survived due to a failure of the ventilation system.
Will all of this turn out to be a problem for astronauts on long-duration spaceflights? To me, it seems quite plausible — spaceflight has been shown to affect immune function in other studies. However, this study was performed on amphibians, and while many aspects of immune system function are shared across all jawed vertebrates, it would be a stronger story if the same phenomena were replicated in larger studies and/or those including mammals. Also, a link between decreased antibody diversity and an increased susceptibility to disease would need to be demonstrated (perhaps this has already been done in non-spaceflight scenarios).
Either way, I found this to be an interesting story, and it’ll be really interesting to find out what’s going on at a deeper level!
Article doi: 10.1096/fj.11-185215
Cardiac Arrhythmias and Modeling (Part 2)
In Part 1, I gave an introduction to normal electrical conduction in the heart, and why it’s important. Now, it’s time to look at how things can go wrong…
Arrhythmias: When the Conduction System Goes Awry
First of all, a word of reassurance: most of us will live full lives without experiencing any arrythmias, or at least being aware that one has occurred. The cardiac conduction system is very reliable and works well under changing conditions, day in and day out, year after year. While most arrhythmias are not harmful, there are some that are serious, or even lethal.
Arrhythmias can generally be placed into one of three groups: slow, fast, or irregular. I won’t provide an exhaustive list of all arrhythmias here; I just want to provide a few examples. Also, just as a reminder, here’s what a normal ECG looks like:
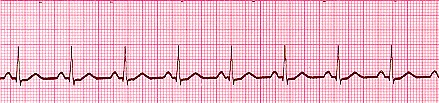
Image Credit: ambulance technician study
Slow Rhythms
Slow arrhythmias are called bradycardias, and are generally caused by problems with either the SAN or the AVN (SAN = sinoatrial node, AVN = atrioventricular node, see Part 1). Below is an example of a severe bradycardia, third-degree heart block, which is often problematic because the result is a heart that is pumping too slowly to maintain adequate blood pressure. You’ll notice two things about this ECG strip. First, the P-waves are completely dissociated from the QRS complexes: the distance between a P-wave and the QRS complex that follows it changes with each beat and follows no pattern. This is because signals that are being generated in the SAN and traveling through the atria down to the AVN are completely blocked upon arrival at the AVN. Thus, there is the normal activity up in the atria, producing regularly spaced P-waves, and there is activation of the ventricles, producing regularly spaced QRS complexes, but there is no relationship between the two, owing to the complete block of signals at the AVN.

Image Credit: ambulance technician study
Second, the QRS complexes are much wider than those shown above in the “normal” example. Most cardiac tissue has some ability to act as a pacemaker. Here, the ventricles are being excited by a signal originating from somewhere below the AVN, at a rate slower than the P-waves which are being generated by the SAN. Also, the fact that the ventricles are responding to a signal that originates from somewhere other than the SAN means that electrical activation within the ventricles follows a different pathway, which makes the QRS complexes look “wide and bizarre.”
Fast Rhythms
Fast arrhythmias are called tachycardias. Below is an example of a particularly serious, and potentially lethal, tachycardia: ventricular tachycardia. Ventricular tachycardia is dangerous for two reasons: 1) It results in the ventricles beating so fast that they don’t have time to fill in between beats, which severely limits the ability to pump blood, and 2) it can degrade into an even more disorganized rhythm: ventricular fibrillation.

Image Credit: ambulance technician study
Irregular Rhythmis (wait, is than an oxymoron?)
One type of arrhythmia in the “irregular” class is fibrillation. Returning to the orchestra analogy from the last post, fibrillation is akin to what you hear before the start of an orchestra’s performance: random jumbles of sound as each musician practices and tunes on their own. When a heart is fibrillating, each little region is being activated on its own and is not responding to directions from any sort of pacemaker. This highly disorganized pattern of electrical activation results in a heart that quivers, rather than doing any sort of organized pumping. It is a common occurrence for the atria to fibrillate, especially as people age, a condition called (unsurprisingly) atrial fibrillation. Atrial fibrillation can cause a reduction in the heart’s pumping ability, since the ventricles are not being as fully loaded as they could be before they contract and force blood out to the rest of the body. Atrial fibrillation is also problematic because it can lead to the development of blood clots. If the atria are just sitting there quivering, blood may sit in one corner for too long, which promotes clotting. Eventually the clotted blood finds its way down into the left ventricle, where it is then pumped out to the rest of the body. If such a clot lodges in one of the brain’s blood vessels, a stroke can occur. Below is an example of atrial fibrillation. You’ll notice that there is “messy” electrical activity in between QRS complexes (which are themselves occurring irregularly), as opposed a clean baseline and distinct P-waves.
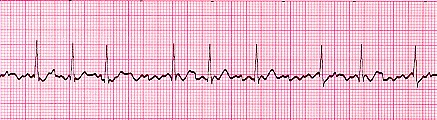
Image Credit: ambulance technician study
Ventricular fibrillation, as you can imagine, is much more dangerous, as no blood is being pumped anywhere. The only way to correct V-fib is to apply a large electrical shock (just like when you see a patient being shocked with paddles on television). In the ECG strip above, there was some disorganized electrical activity because the atria were fibrillating, but the ventricles were still producing distinct QRS complexes. However, there are no QRS complexes in the ECG strip below since the entire heart is fibrillating.

Image Credit: ambulance technician study
Another type of arrhythmia in the “irregular” class is the premature complex. A region of heart tissue can become excited on its own, and if this region is large enough, the resulting activation can propagate through the entire heart resulting in a complex (or contraction) before one would be expected from the dominant pacemaker site. These early activations, if originating from somewhere withing the ventricles, are called premature ventricular complexes (PVCs). Now, PVCs on their own are fairly benign, but they can be worrisome for two reasons. First, they often signify some underlying problem that’s manifesting itself as an abnormality with the heart’s electrical system. Second, it’s possible for a PVC to occur during a “vulnerable window” and trigger something called a reentrant arrhythmia, one example being ventricular tachycardia.

Image Credit: ambulance technician study
There are two PVCs in the above ECG, occurring after the second and fifth normal beats. These are multifocal PVCs – they originate from different locations within the ventricles – which means that the resulting activation follows a different path in space for each one, and is why the two PVCs look so different from one another.
Reentry: A “Short Circuit” Within the Heart
Reentry is a situation where some region of cardiac tissue is repeatedly (and rapidly) excited as an electrical activation wave travels round and round, following a circular path. Once cardiac tissue has been excited, there is a period of time, called the refractory period, which must pass before the tissue is able to be excited again. What this means is that normally electrical activation follows a path in one direction only; it cannot turn around and go back from where it came because the tissue at the trailing edge of the activation wave is still refractory. A more detailed description of reentry can be found here. I mention reentry because certain tachycardias (including ventricular tachycardia, mentioned above) and fibrillation (also mentioned above) are examples of reentrant arrhythmias.
Okay, so some particularly nasty arrhythmias result from reentrant pathways in the heart. But what causes reentry in the first place? There are two conditions, often working in concert, that greatly increase the chances of a reentrant pathway forming. One of these conditions is slowed conduction. If electrical activation travels more slowly in one region of heart tissue than another, this can help set the stage for reentry. The second condition is referred to as unidirectional block. Electrical activation in the heart doesn’t occur in one-dimensional lines, but rather spreads in multiple directions. If the spread of an electrical wave is blocked in one direction, but not the other, this can facilitate the development of reentry.
Now we’re moving into increasingly detailed explanations of some causes of cardiac arrhythmias. Given that some arrhythmias are manifestations of reentry, and reentry can be established by slowed conduction and/or unidirectional block, what’s responsible for slowing conduction, or creating a unidirectional block? Remember those PVCs I was talking about earlier? They (or even smaller regions of depolarization that don’t manifest as PVCs) can create a unidirectional block. I mentioned previously that cardiac tissue has a refractory period during which it is impossible (or much more difficult) to be excited again. If a PVC occurs in one region of cardiac tissue, that region will now be refractory. Now imagine a spreading activation wave, and what would happen if one part of that wave tried to spread into a region that had just been excited by a PVC. Since the tissue is now refractory (thanks to the PVC), the spreading wave will be blocked in that direction, but will still be able to spread in another direction into tissue that isn’t refractory. Voilà! You now have unidirectional block.
If you want to get a better idea of what I’m talking about, take a look at the animation at the top of Frank Starmer’s page, who is a researcher at Duke University. The animation represents a two-dimensional sheet of excitable cells, with yellow representing non-excited cells and red representing excited cells. At the start, a planar wave (red line spanning the entire yellow sheet) is initiated and propagates downward. Shortly thereafter, a premature stimulus is delivered (shorter red line near the top of the sheet). This premature stimulus cannot propagate downward, as the first wave has just passed through that region, leaving those cells in their refractory period. But the tissue above the premature stimulus has had enough time to recover from its refractory period, and so the premature stimulus moves upward but not downward. This unidirectional block sets up the spiral wave activity that you see in the last half of the animation.
Ultimately, many of the explanations for what causes slowed conduction or unidirectional block (including what causes a PVC to occur) are found at the level of individual cardiac cells, or even subcellular components. Single-cell descriptions can take us only so far, but they are still very powerful. In Part 3, I’ll talk about how we use computers to simulate individual cardiac cells, as well as larger regions of tissue.
Cardiac Arrhythmias and Modeling (Part 1)
When someone learns that I’m a grad student, the next question is invariably, “So what do you work on?” My answer varies depending on who’s asking, but usually includes something along the lines of:
1. “I’m interested in cardiac arrhythmias.”
2. “I do computer modeling of the heart.”
In an attempt to provide a better answer (and provide some relevant background) than I’m usually able to in the time available, I’m going to dedicate a few posts to the subjects of cardiac arrhythmias and why/how we use computers to study them.
But before I describe what an arrhythmia is (and why it’s potentially deadly), I need to first spend a little bit of time talking about normal electrical activity in the heart…
An Introduction to Cardiac Conduction: The Heart as an Orchestra
The purpose of the heart is, of course, to pump blood throughout our bodies. But in order to do this, the millions of cells that make up a heart must work together and contract in a prescribed pattern, both in time and space. Muscle cells contract in response to electrical stimulation, which means that in order to get the correct pattern of muscle contraction, the correct electrical pattern must be generated.
Many cells in the heart have the ability to excite themselves. However, if each cell did its own thing, the heart would be reduced to a quivering, non-pumping liability, not an efficient pumping machine. In order to generate the necessary organized patterns of electrical activation, the collective of heart muscle cells must receive direction. Much as the individual musicians of an orchestra generate beautiful music under the direction of their conductor, cardiac cells too have a conductor, called the sinoatrial node (SAN) (This is not to say that said musicians can’t generate beautiful music individually; I trust that you get my point.) As shown in the figure below, the SAN, which sits high up in the right atrium, generates an electrical signal that spreads throughout the two atria (upper chambers of the heart) and down to the atrioventricular node (AVN). As the wave of electrical activation spreads throughout the right and left atria, the atrial cells contract, pumping blood down into the respective ventricles.

Image credit: Richard Klabunde
When the wave of electrical activation reaches the AVN, it’s held up briefly before being allowed to proceed. This is to allow enough time for the ventricles to be filled with blood before they contract and pump blood to the rest of the body. From the AVN, electrical activation continues along the pathways shown in green above, traveling down the bundle of His, then divided between the right and left bundle branches, and eventually passing into the increasingly branched Purkinje system, leading to the excitation and contraction of cells in the ventricles.
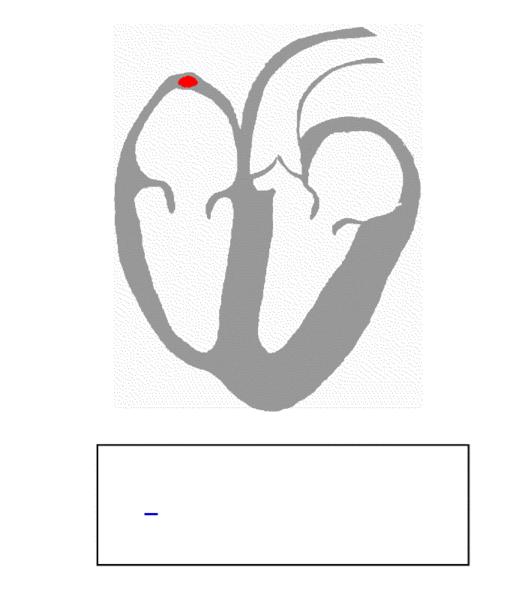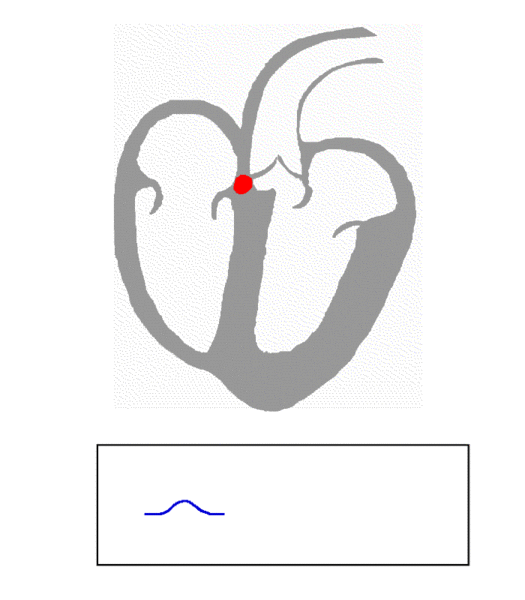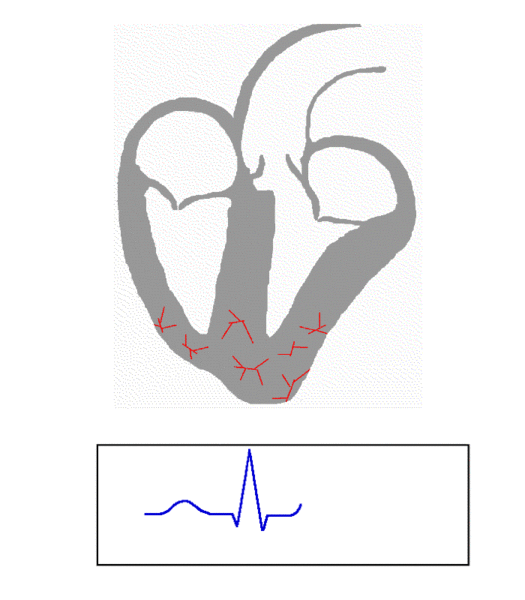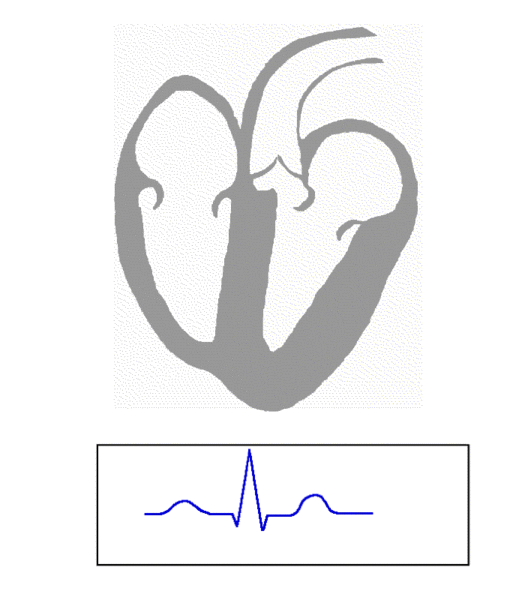
The animation below summarizes all of this: electrical activity originating at the SAN (upper left region of the image), spreading through both atria, passing through the AVN, then down the septum that separates the two ventricles and up the ventricular walls. Immediately following the electrical activation (also known as depolarization, shown in red), the corresponding heart muscle (shown in gray) contracts. You’ll also notice that after the ventricles contract there is a second wave of electrical activity (this time repolarization, as opposed to depolarization, also shown in red) that leads to muscle relaxation.
Image Credit: Wikimedia Commons
The blue tracing at the bottom of the animation is a simulated electrocardiogram (ECG) being generated as electrical excitation spreads throughout the heart. Here’s a schematic:
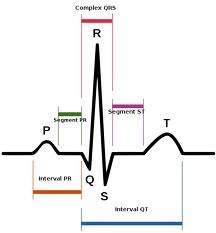
Image credit: Wikimedia Commons
An ECG is simply a recording of changes in voltage across the heart over time. Starting from the left, at the beginning of a beat, the P-wave corresponds to electrical activation (depolarization) of the two atria. A little bit later, the QRS complex is generated, which corresponds to depolarization of the ventricles. Following that is repolarization of the ventricles, which is represented by the T-wave. You’re probably asking where repolarization of the atria fits in. This occurs at about the same time as the ventricles are contracting, and as such is “hidden” in the QRS complex. Here’s an example of a normal ECG strip, showing four beats:

Image Credit: The Student Nurse Forum
In Part 2, I’ll discuss some ways in which normal cardiac conduction can be disrupted (which is where the part about arrhythmias comes in), and provide some examples that significantly differ from the normal ECG above.
What is a Space Agent, Anyway?

Image credit: Virgin Galactic, LLC
If you were to book a flight to space aboard Virgin Galactic’s SpaceShip Two today, you’d likely do it through one of their Accredited Space Agents. But what IS a space agent? I have a story over at the Space Future Journal that explains what sets a space agent apart from a regular travel agent, and why you might consider using one. Check it out!
VSS Enterprise Makes Its First Piloted Free Flight
Congratulations are definitely in order for Virgin Galactic and Scaled Composites! Yesterday marked a successful first piloted free flight of the VSS Enterprise, also known as SpaceShipTwo.
The Enterprise was carried to an altitude of 45,000 feet by its mother ship, WhiteKnightTwo (VMS Eve), and released over the Mojave desert. From there, it glided down and made a beautiful landing (as seen in the embedded video from Virgin Galactic’s YouTube site below), with pilot Pete Siebold and co-pilot Mike Alsbury at the controls.
Virgin Galactic, which is poised to be the first company to take passengers into space on a regular basis, is employing Burt Rutan’s Scaled Composites to develop the mother ship and spacecraft, which will carry six passengers and two pilots into suborbital space.
Update:
Moshe Gabbay, one of Virgin Galactic’s Accredited Space Agents, emailed me these spectacular photos this morning:





More About Me
There’s a short blurb in my “About” section, but I wanted to create a post that contains a little more information about my background and the circuitous path to where I’m at now. I believe it can be useful for readers to know something about their bloggers, not to mention writing such a post feeds my narcissism (kidding!… well sort of). So if we don’t already know each other, you’re curious, and you have the time to spare, read on!
I grew up in Sonoma County, often known as a wine region about an hour north of San Francisco. Contrary to what many people expect, I know relatively little about wine. My mother worked at Korbel (known more for their champagne and brandy than their wine), and I even worked as a “crush technician” at Korbel for a summer, but I suppose that growing up surrounded by something can lead one to take it for granted, or even to eschew it. As far back as I can remember, I was interested in gadgets and learning how things work. Before I figured out how to use tools to take things apart, I used to smash them apart so I could get a peek at what was inside. When I was 5 or 6, my father gave me an old record player that he had for many ears. Of course, I ultimately smashed it to bits so I could see what made it tick. When my father learned what I had done, he was livid. According to him, when he asked me why I had destroyed the recored player, I matter-of-factly replied that I wanted to see what was inside.
In later years I became fascinated with computers, learning to type on an Apple IIe in junior high and teaching myself a little BASIC. My first computer was a Texas Instruments TI-99/4A, on which I spent many hours playing Tunnels of Doom (a game whose awesomeness was evidenced by the fact that its code could not fit within the paltry space available on a game cartridge; you had to load additional data from/to an audio cassette). I later decided that I didn’t want to become a computer programmer, as I found debugging computer programs to be maddening (ironic, given my current work!)
I developed an interest in medicine during my late teen years. When I was 16, I became a volunteer firefighter (my father was a fireman, and at that time, at our small-town fire department, you could sign up as a volunteer when you turned 16). Unlike my brother, I didn’t have any significant aspirations to become a firefighter; it just seemed like the thing to do at the time since I’d grown up around it. In many fire districts, the majority of calls are not for fires, but for medical assists, so I saw a lot of patients with breathing difficulties, diabetic emergencies, various types of pain and minor trauma, etc. in addition to vehicle accidents, rescues, and fires. One of my first patients was a guy who had lacerated his arm pretty severely by putting it through a glass window while he was fighting with his girlfriend. As I was bandaging him, I realized that medicine was pretty cool: you could learn a lot about science and the human body, and help people at the same time. When I turned 18, I became an Emergency Medical Technician and soon thereafter began volunteering in the emergency room at the local hospital. I spent a LOT of time in that hospital, mostly in the ER, but I also stayed up all night on many occasions watching surgery or following interesting cases in the ICU.
When I graduated high school, I started taking classes at Santa Rosa Junior College. I knew that I was interested in going to medical school, but wasn’t really ready to move to a university. Not being a very good student, i spent a couple years at the Junior College taking classes, spending too much time in the hospital (which was way more fun than doing homework) and getting mediocre grades.
After a while, I finally completed enough work to make me eligible to transfer to a University of California school, but instead of doing so, I decided to go to paramedic school. At this point I’d decided that I wanted a career in emergency medicine, so one motivation for becoming a paramedic was to prove to myself that I had what it takes to succeed in emergency medicine. As a paramedic, you frequently have to figure out what’s wrong with a patient based upon very little information, successfully come up with a treatment plan and execute it, do so relatively quickly, and with no one more knowledgable than you on the scene to back you up. A second benefit was that the pay would be better than what I made working in pizza restaurants, making it easier and more enjoyable to work my way through college. Even after five years of graduate school, I still contend that paramedic school was the hardest and most stressful thing that I’ve ever done. Paramedic training was similar to grad school in that I had to read a lot and rapidly learn about topics with which I had little familiarity, but on top of that I had to make sure that I didn’t accidentally kill my patients, and try not to get sued.
After working for a couple years on 24-hour shifts, I was able to score a 12-hour night shift gig which allowed me to go back to school. I returned to Santa Rosa Junior College for a little over a year to finish some liberal arts requirements and to re-take organic chemistry (which I had barely passed and learned nothing of the first time around). I’d go to class during the day, go home for a couple of hours to nap, then work a 7pm-7am shift in the 911 system (I learned a lot about SN1 and SN2 reactions while sitting in the front of an ambulance between calls!). This time, however, I was a much better student: I had recently re-discovered my love of science (seeded by a Scientific American article about Hedgehog genes), in addition to the fact that being away from school (and returning voluntarily) greatly enhances your appreciation for being in college. In 2001 I transferred to UC Davis as a Cell Biology major.
Davis was where my transition from clinical medicine to science really took place. During my first quarter there, I asked one of my professors about what sort of preparation I would receive in medical school for doing scientific research. He told me that I should apply to a PhD program, complete two years, then leave with a Masters and go to medical school. Probably not the advice that I would’ve given, but it nonetheless was the first time that I gave any consideration whatsoever to applying to a PhD program. At this time I had also started doing research in a C. elegans lab, helping a graduate student who was trying to clone a gene necessary for proper orientation of the mitotic spindle during cell division. By now, the years of working in emergency medicine were finally taking their toll, and I was coming to the realization that a career in clinical medicine no longer appealed to me. So by the time I entered my second quarter, I’d decided that while I would miss some aspects of taking care of patients (and still do), I’d rather make my contributions to medicine through research.
After two years at UCD I graduated with a B.S. in Genetics, then took a job as a postgraduate researcher in a brain cancer lab. This position was appealing to me, as my interests in biomedical research are largely motivated by my past experience in the clinical world. I was hired by two veterinary neurosurgeons who, in addition to carrying out basic research into the molecular biology of brain tumors, also diagnosed and treated brain tumors in dogs and cats who were brought into the clinic by their owners. It turns out that canine brain tumors are very similar to human brain tumors in many ways: genetic and structural changes as well as response to therapy. Therefore, in addition to benefitting the animals that presented to the clinic, we could potentially benefit human patients down the road. This new job was in many ways ideal for me: I split my time between the lab and a hospital setting, since part of my job was to be present in the operating room to receive samples of brain tumors that were biopsied, as well as assist with and perform brain imaging. Some of my work was more basic science in nature, such as sequencing tumor samples to look for DNA mutations and doing cell culture, but some of my work was also translational, such as developing improved therapies for the treatment of brain cancer, as well as improved methods for developing therapy.
During my last year of college, I was introduced to the various ‘omic’ fields: genomics, proteomics, etc. I also became deeply interested in the idea of systems biology, and in particular network biology, as a way to better understand the mechanisms of disease and to more intelligently develop therapies to treat and prevent disease. It also became clear to me that the types of problems I was interested in would require computational approaches, yet I had no computational or mathematical background. Therefore, when I began to apply to PhD programs, I was on the lookout for computational biology programs (of which only a couple existed at the time) or programs that would allow me to increase my knowledge of computer science and mathematics. I wasn’t interested in becoming a computer scientist per se, but I also didn’t want to be someone who handed off problems to computer scientists and say, “here, do this analysis for me.” I wanted to be able to “talk the talk” with computer scientists, at least well enough to work as a team in figuring out how to tackle the problems I was interested in.
So that’s how I ended up at where I am today. In 2005 I was accepted into the Tri-Institutional Training Program in Computational Biology and Medicine, which counted Cornell University (both the main campus in Ithaca and the medical campus in NYC), the Memorial Sloan-Kettering Cancer Center, and The Rockefeller University as participating institutions. My thesis work has been been in the area of computational cardiology, building and using computer models of heart cells and tissue to better understand cardiac arrhythmias (abnormal electrical patterns in the heart). You’ll certainly hear more about this in future posts.
Hopefully this rather long-winded story of my life will leave you feeling like you know me a bit better, and prove useful for understanding why I’m interested in some of the things that I am, as well as providing context for my commentary in the future….
Astronaut Centrifuge Training: A thing of the past… or future?

During a tour of the Johnson Space Center last summer, I was surprised to learn that NASA astronauts no longer participate in centrifuge training (at least not for Shuttle missions). This led to a brief story that is now up over at the Space Future Journal. Check it out!
Image credit: Lance Cheung
Something to Hold You Over…
Welcome! Until I get something new written, here’s an article that I wrote about cardiac dynamics over at the old host of this blog, Scientific Blogging: The Heart: So Elegant, Yet So Complex.